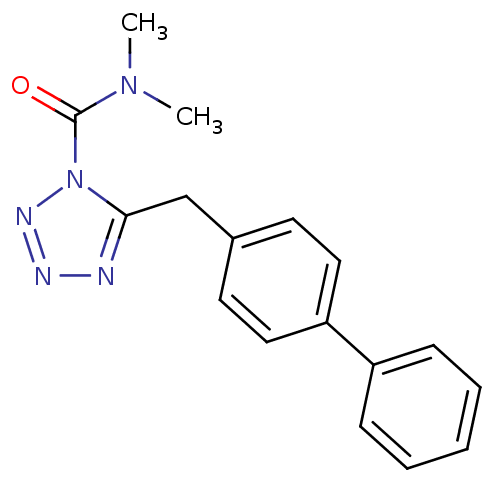
BDBM26736 CHEMBL509860::LY2183240::N,N-dimethyl-5-[(4-phenylphenyl)methyl]-1H-1,2,3,4-tetrazole-1-carboxamide
SMILES CN(C)C(=O)n1nnnc1Cc1ccc(cc1)-c1ccccc1
InChI Key InChIKey=GZNIYOXWFCDBBJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 26736
Found 4 hits for monomerid = 26736
Affinity DataIC50: 54.4nMAssay Description:Inhibition of human MGLMore data for this Ligand-Target Pair
Affinity DataIC50: 290nMAssay Description:Inhibition of human recombinant MAGL-mediated 1,3-dihydroxypropan-1-yl 4-pyren-1-ylbutanoate conversion to 4-pyren-1-ylbutanoic acid preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 55.0nMAssay Description:Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 5.30nMpH: 7.4 T: 2°CAssay Description:Serine hydrolase targets were recombinantly expressed in COS-7 cells by transient transfection. IC50 values were obtained by competitive ABPP with F...More data for this Ligand-Target Pair